How Roll-to-Roll Coating Parameters Impact Lithium-Ion Battery Performance
Smarter Film Formation in Battery Manufacturing: What a New Study Tells Us
Can precise control over speed and pressure improve battery performance? A recent study titled "Identification of Cause–Effect Relationships between Process Parameters and the Film Formation in the Semidry Electrode Production for Lithium-Ion Batteries" answers this question with fresh insights. Researchers from the Technical University of Munich and Fraunhofer IGCV have investigated how variations in calender gap and roller speed influence the quality of electrodes for lithium-ion batteries.
Traditional electrode production methods rely heavily on solvents, which increases both energy demand and equipment size. The semidry method addresses this by cutting solvent use by more than half. This makes the process more energy-efficient and space-saving. However, to bring this method to large-scale production, manufacturers must understand how specific parameters affect the final electrode properties.
This study identifies those crucial cause and effect relationships. It provides clear guidance for manufacturers aiming to improve both efficiency and battery performance.
Key Highlights
Semidry electrode production reduces solvent content by more than 50 percent
Calender gap has a strong influence on mass loading and electrode thickness
Roller speed affects ionic resistance more than previously believed
Higher roller speeds result in lower ionic resistance
Silicone residues from the release foil were found on electrode surfaces
Larger granules increased density but decreased surface uniformity
Semidry electrodes outperformed conventional electrodes in ionic resistance
Mechanical strength slightly decreased at higher roller speeds
The Laboratory Roll-to-Roll Coater is the ideal coating machine for scaling up to roll-to-roll processing of battery electrode materials or for testing and optimizing battery electrode slurries.
What Is Semidry Electrode Production and Why Does It Matter?
Semidry electrode production is a promising alternative to traditional slurry-based methods. Instead of using a high-solvent slurry, the electrode materials are processed into granules. These granules are fed directly into a calender where they are pressed onto a current collector while also drying in place.
This approach combines multiple steps including coating, drying, and calendering into a single efficient operation. It significantly reduces energy use by minimizing solvent evaporation. In addition, the method is compatible with conventional binders such as CMC and NBR, allowing manufacturers to adopt it without a complete overhaul of their material systems.
Because it improves efficiency while maintaining or improving performance, semidry production is gaining serious attention as the industry looks for scalable and sustainable solutions.
The Significance of the Study: Why Understanding Process Parameters Is Essential
Despite its advantages, semidry electrode production is still in the pilot phase. To bring it into widespread use, manufacturers must understand how changes in process parameters impact the resulting electrodes.
This study shifts the focus from granule preparation to the film formation stage. It analyzes how calender gap and roller speed influence properties such as thickness, porosity, and ionic resistance. These factors are essential for battery performance.
With this knowledge, manufacturers can precisely adjust production settings to optimize the trade-offs between density, conductivity, and mechanical strength. The result is a more predictable and controllable process that leads to higher-quality battery cells.
The Slot-die Coater is an excellent choice for researchers focused on developing and optimizing battery electrode slurries at the laboratory scale.
Diving Into the Methods: How the Research Was Conducted
The research team used a structured experimental design to test different combinations of calender gap and roller speed. They produced 24 variations of electrodes by changing the gap between 60 and 110 micrometers and adjusting the roller speed from 1 to 4 meters per minute.
Each electrode was evaluated for thickness, mass loading, porosity, and mechanical hardness. Electrochemical performance was assessed using impedance spectroscopy. Surface analysis using SEM and EDXS helped detect any residue or microstructural inconsistencies.
This comprehensive approach allowed the team to isolate specific effects of the process parameters and identify statistically meaningful relationships.
What the Results Reveal About Film Formation
The results show that calender gap has a strong linear influence on mass loading and electrode thickness. As the gap widens, more material is deposited and compressed onto the current collector. This leads to thicker and denser electrodes with less porosity.
Roller speed also plays an important role, especially at smaller calender gaps. Higher speeds resulted in greater mass loading and lower porosity, although the relationship was nonlinear. The study suggests that friction and shear forces between granules and rollers change with speed, affecting how material feeds into the calender.
Electrodes produced at higher speeds also demonstrated significantly lower ionic resistance. This was true even when mass loading and porosity remained constant. The findings suggest that faster roller speeds lead to more efficient particle packing, which facilitates ion movement during battery operation.
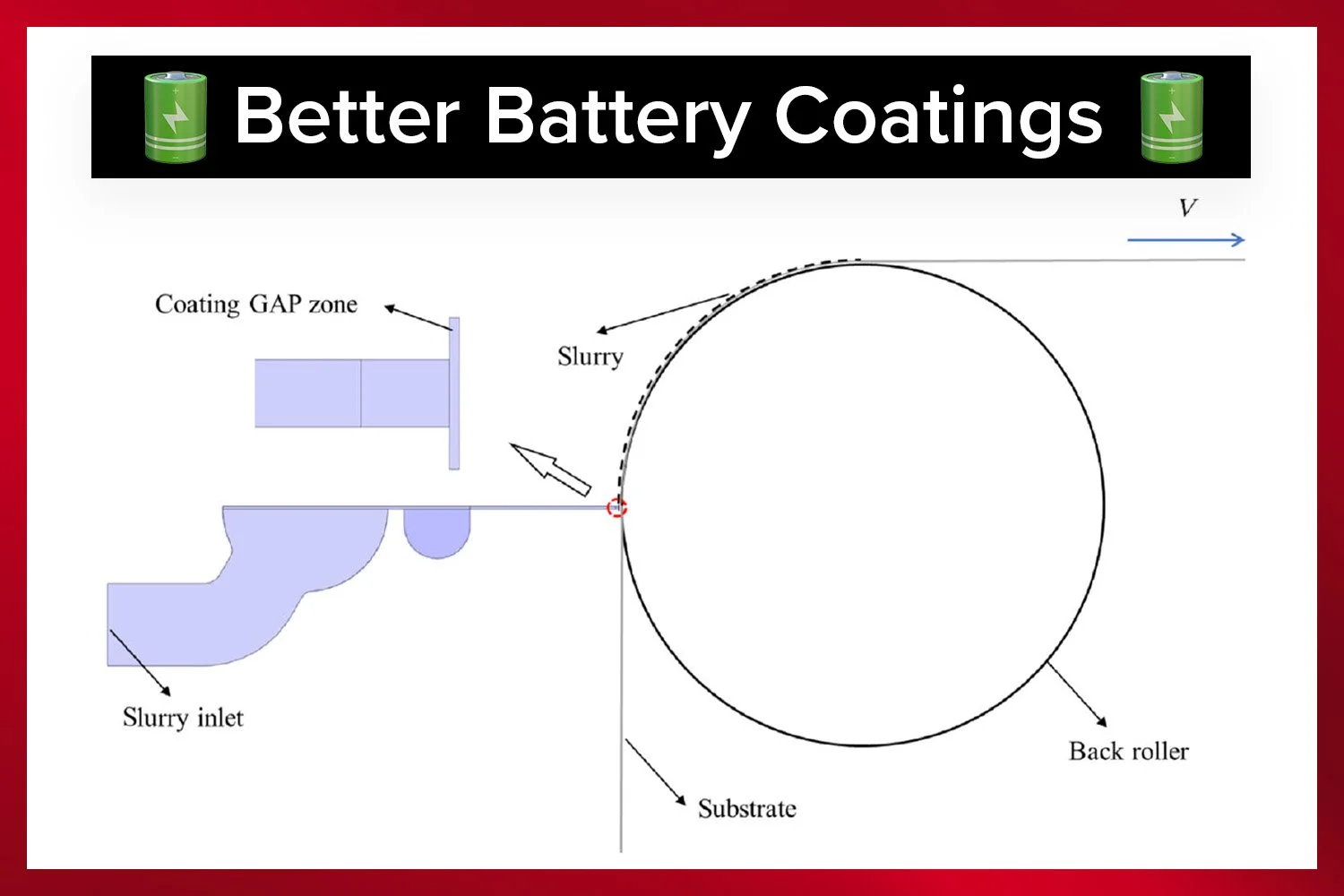
Double-sided battery coating on a Laboratory Roll-to-Roll Coater.
Surface Residues and Their Effects
One unexpected finding was the presence of silicone residues on the surface of the electrodes. These residues came from the silicone-coated release foil used in the process. They were more prominent at lower roller speeds due to longer contact time between the foil and electrode.
Although the total silicon content was low, under 1 percent, it had measurable effects on electrochemical and mechanical properties. SEM and EDXS analyses confirmed that these residues were unevenly distributed and correlated with roller speed.
Interestingly, even with these surface residues, semidry electrodes still outperformed conventional wet-coated electrodes in terms of ionic resistance. This suggests that the benefits of the semidry method outweigh the drawbacks of minor surface contamination. However, the choice of release foil should be carefully considered in future implementations.
Mechanical Properties and Process Effects
Mechanical testing using nanoindentation showed that the hardness and reduced elastic modulus of the electrodes decreased slightly at higher roller speeds. This is likely due to the softer nature of the silicone residues present on the surface.
Despite this, the internal structure of the electrodes remained strong. The minor changes in surface hardness did not compromise the functional performance of the electrodes. The researchers suggest that with optimized release materials, these effects could be minimized further.
Looking Forward: Industrial Relevance and Next Steps
This study provides essential data for battery manufacturers considering semidry electrode production. By quantifying the effects of calender gap and roller speed, it offers a clear framework for optimizing the process in real-world production environments.
The findings also suggest that manufacturers should not overlook roller speed as a key variable. It influences not only productivity but also electrode performance. As the industry seeks ways to scale up production while cutting energy use, semidry processing stands out as a viable path forward.
Future research should investigate alternative release foils to eliminate surface contamination. Additional studies could also explore broader ranges of granule sizes and calender configurations to fine-tune the process even further.
Conclusion
This study demonstrates how fine-tuning calender gap and roller speed can significantly improve the quality of semidry-produced lithium-ion battery electrodes. It confirms that semidry processes can deliver better electrochemical performance than conventional methods while also reducing energy use.
The role of roller speed, in particular, emerged as more important than previously assumed. It directly affects ionic resistance and may even influence mechanical properties through subtle surface effects.
By understanding these relationships, manufacturers can take a major step toward more efficient, scalable, and sustainable battery production. Semidry electrode technology is no longer just a concept — it is rapidly becoming a practical solution for the future of energy storage.
Authors
Matthias Leeb
Nico Schwarz
Rüdiger Daub
Get Professional Support for Your Battery Coating Needs
Need help with slot-die coating, coating machines, or any related applications?
Contact infinityPV’s experts today for professional guidance and support.






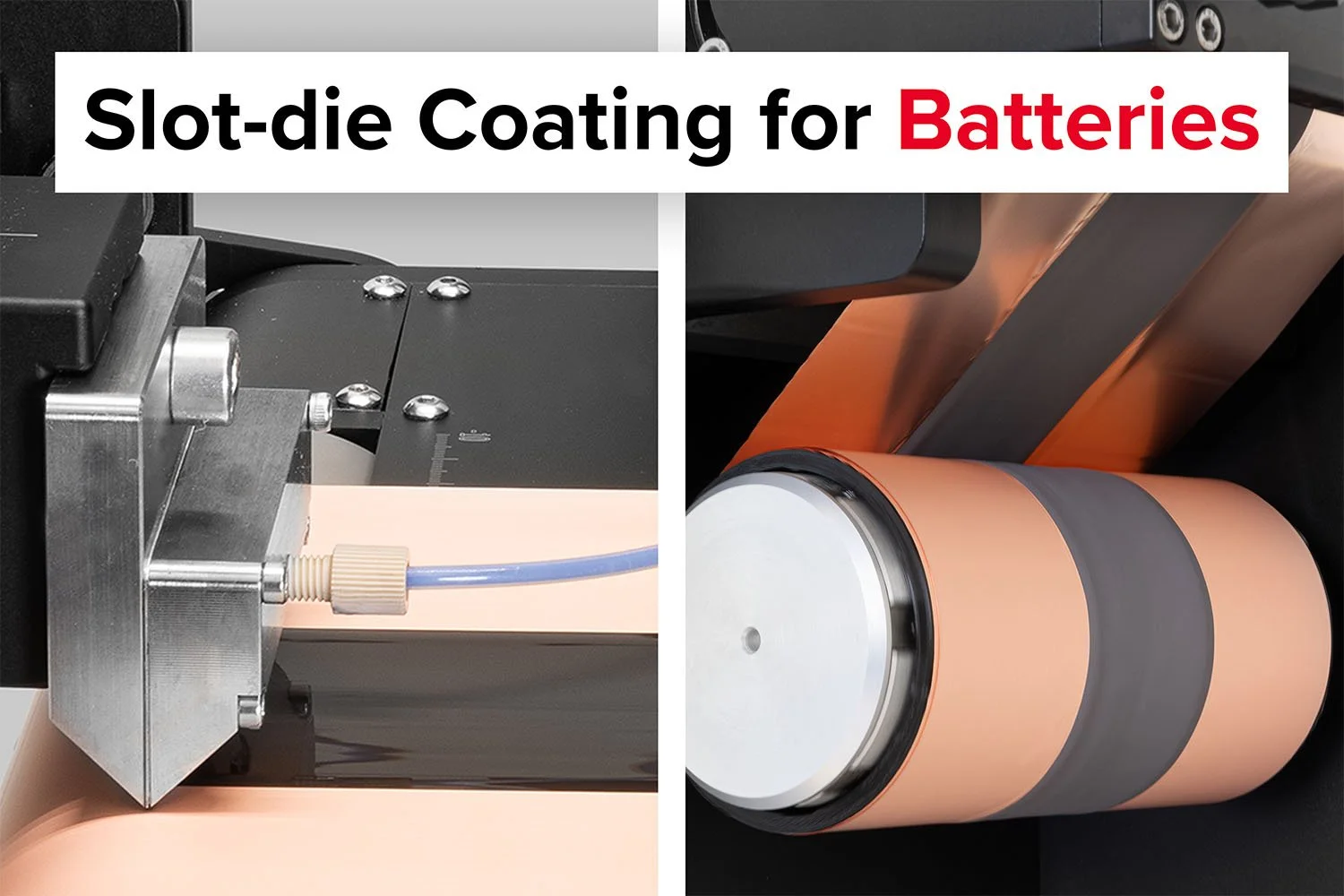













Probably the World’s Most Compact R2R Slot-die Coater: A compact, fully integrated roll-to-roll coating platform for laboratories, complete with a mounting system, anodized rollers, a syringe pump, a 65 mm stainless slot-die head and an infrared oven system—delivering unmatched precision and scalability.