Silicon Anodes Breakthrough: Dry Electrode Processing Meets Stability
Can Silicon Anodes Finally Deliver on Their Promise?
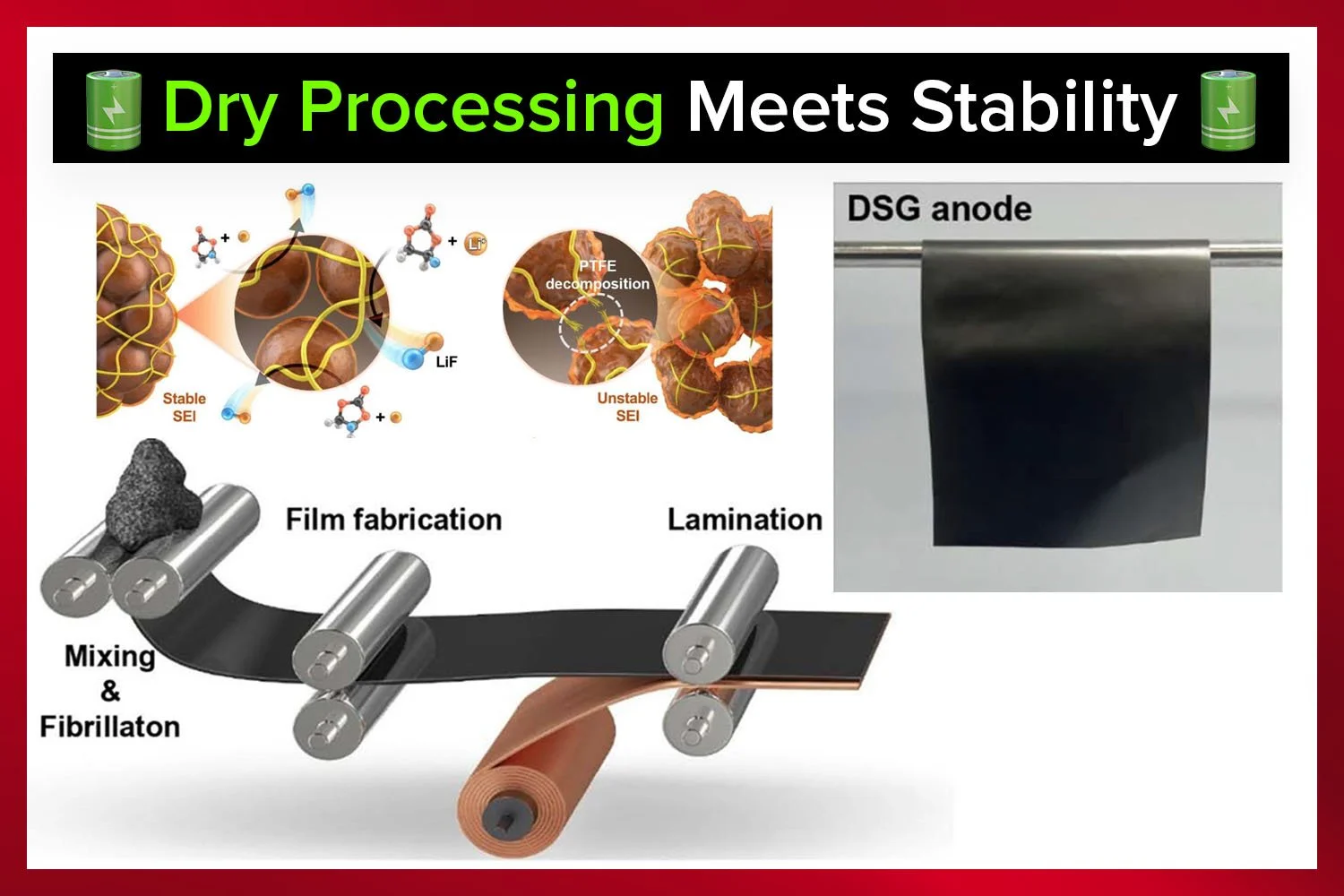
Silicon anodes have long been viewed as the most promising alternative to graphite for lithium-ion batteries. With nearly ten times the theoretical capacity of graphite, silicon could unlock electric vehicles with longer driving ranges and faster charging speeds. The challenge, however, has been durability. Silicon expands and contracts by as much as 300 percent during charge and discharge cycles, which causes electrode cracking, loss of contact, and rapid capacity fade. At the same time, a new way of manufacturing electrodes known as dry processing is gaining attention because it avoids toxic solvents and simplifies manufacturing. Yet dry processing also has its own problem: the PTFE binder used in these electrodes tends to decompose during cycling, weakening the mechanical stability of the electrode and reducing efficiency.
The recent study, "Tailored electrolyte additive design for suppressing irreversibility in dry-processed anodes and enhancing electrochemical stability in full-cells" (Han et al., 2025), directly addresses both of these challenges. The researchers explored the use of fluoroethylene carbonate (FEC) as an electrolyte additive in dry-processed silicon/carbon–graphite (DSG) anodes paired with Ni-rich cathodes. By systematically testing different concentrations, they identified an optimized level of 10 wt% FEC that stabilized the anode, reduced binder breakdown, and avoided cathode degradation. With this optimized system, the cells achieved exceptionally high coulombic efficiency, long-term cycling stability, and high areal capacity. These results show a practical way forward for dry-processed, silicon-rich anodes that could help commercialize next-generation electric vehicle batteries.
Key Highlights
Silicon anodes offer 10× higher capacity than graphite but suffer from severe volume changes.
Dry electrode processing avoids harmful solvents but suffers from PTFE binder decomposition.
Fluoroethylene carbonate (FEC) additive forms a stable, protective SEI layer on the anode.
Optimal concentration identified: 10 wt% FEC (called FA10).
Too little FEC leads to unstable anodes, too much FEC leads to degraded cathodes and sluggish ion transport.
FA10 delivers 99.9% average coulombic efficiency over 300 cycles in full cells.
High areal capacity achieved: 4.6 mAh cm−2 after 300 cycles.
Post-mortem analysis confirmed reduced binder breakdown at the anode and suppressed HF attack at the cathode.
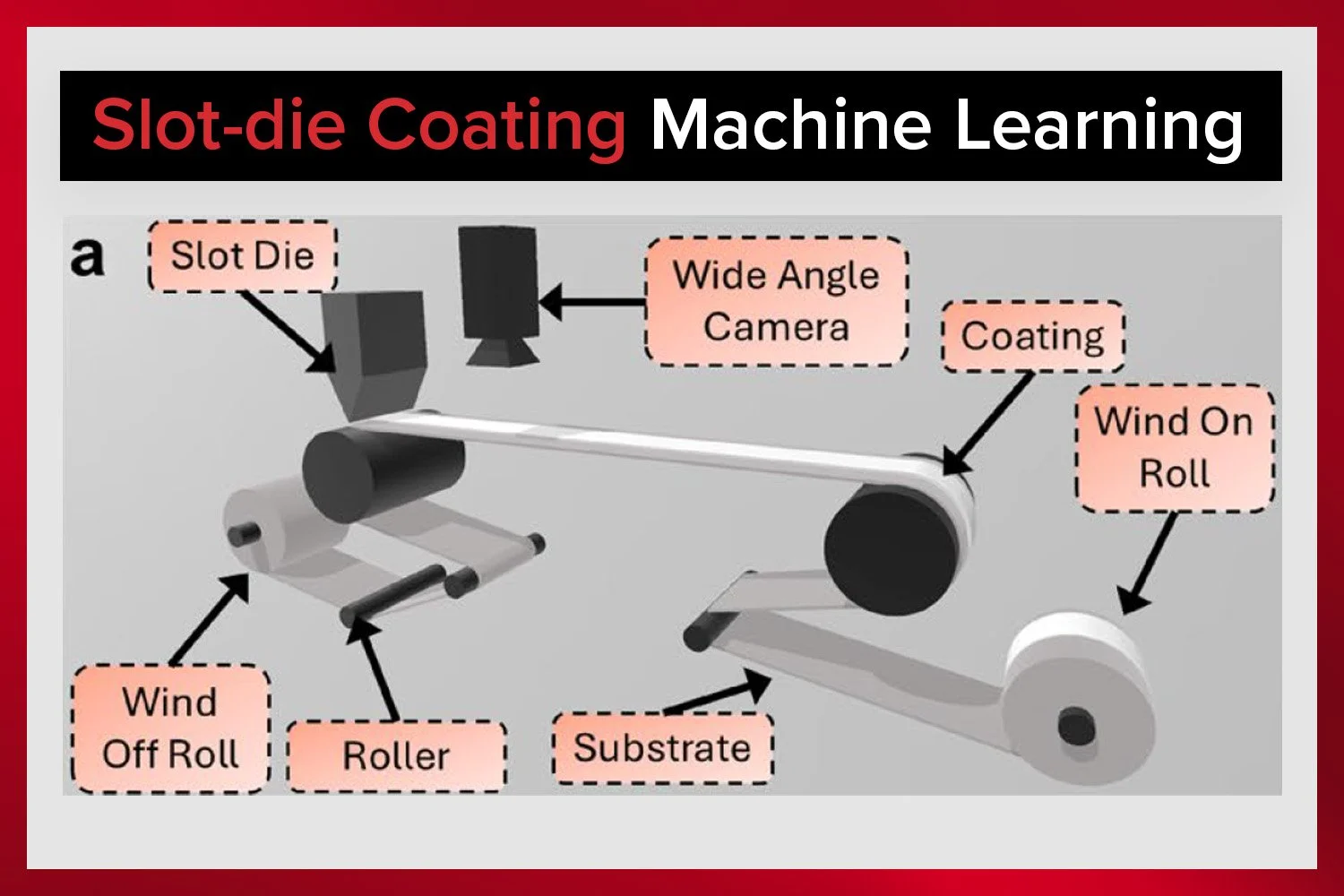
The Laboratory Roll-to-Roll Coater is the ideal coating machine for scaling up to roll-to-roll processing of battery electrode materials or for testing and optimizing battery electrode slurries.
What is Dry Electrode Technology and Why Does it Matter?
In conventional electrode manufacturing, powders of active material, conductive additives, and binders are mixed into a slurry with a solvent. This slurry is coated onto metal foils, dried in large ovens, and then pressed to the correct density. While effective, the process requires costly drying equipment, consumes large amounts of energy, and relies on toxic solvents that must be carefully recovered. This adds both cost and environmental impact.
Dry electrode processing skips the solvent step entirely. Powders are mechanically mixed and pressed into sheets that can be laminated onto current collectors. This solvent-free method makes the process simpler, cheaper, and more environmentally friendly. More importantly, dry electrodes can be manufactured much thicker than slurry-coated electrodes, which is critical for increasing the energy density of batteries. Because of these advantages, dry processing is considered a key technology for future gigafactories.
The drawback is that dry electrodes rely heavily on PTFE binders. PTFE, however, decomposes under the low voltages experienced at the anode, which weakens the electrode and reduces efficiency. Without a way to stabilize this binder and the silicon particles, the promise of dry-processed silicon anodes cannot be realized.
-
1. Non-rechargeable (Primary)
Li-MnO₂ – Used in cameras, medical devices. Long shelf life.
Li-SOCl₂ – Used in meters, sensors. Very high energy, works in extreme temps.
Li-FeS₂ – Found in AA/AAA batteries. Lightweight, good for high-drain devices.
2. Rechargeable (Lithium-ion)
LCO (Lithium Cobalt Oxide) – High energy, used in phones and laptops.
LMO (Lithium Manganese Oxide) – Safer, used in tools and medical devices.
LFP (Lithium Iron Phosphate) – Very safe, long life, common in EVs and solar storage.
NMC (Nickel Manganese Cobalt) – Balanced performance, popular in EVs.
NCA (Nickel Cobalt Aluminum) – High energy and long life, used in Tesla batteries.
LTO (Lithium Titanate) – Super safe, charges fast, lasts very long, but lower ener
The Significance of the Study
The move toward carbon neutrality has accelerated the demand for electric vehicles with longer ranges and shorter charging times. Meeting this demand requires batteries with higher energy densities. Silicon anodes could deliver this performance, but their mechanical instability and the challenges of integrating them into scalable manufacturing processes have been major roadblocks.
This study demonstrates that it is possible to combine high-capacity silicon with dry electrode processing and still achieve durable performance. By tuning the concentration of FEC in the electrolyte, the researchers created conditions where the silicon could expand and contract without destroying the electrode structure. At the same time, the cathode remained protected from degradation. This balance is crucial. Too little additive leaves the anode unstable. Too much additive harms the cathode. The optimized formulation, however, stabilizes both.
This balance is what makes the work significant. It shows not just that FEC works, but how much of it is needed and why. This kind of guideline is invaluable for battery manufacturers who need clear, practical insights that can be applied in scaled production.
The Laboratory Roll-to-Roll Coater makes double-sided slot-die coating simple.
Diving Into the Methods
The research team fabricated dry-processed anodes by mixing a silicon–carbon composite with graphite and pressing it with a PTFE binder into freestanding sheets. These were then paired with Ni-rich NCM cathodes in full-cell configurations. The key experimental variable was the concentration of FEC in the electrolyte: 0, 10, and 20 wt%.
To study performance, the team ran both half-cell tests, where the anode is paired with lithium metal, and full-cell tests, where the anode is paired with a cathode. Half-cells help isolate the behavior of the anode itself, while full cells reveal how the entire battery system behaves. In addition to electrochemical testing, the researchers conducted a wide range of characterizations. SEM and Raman spectroscopy were used to track structural changes in the electrodes. Impedance spectroscopy measured ion transport resistance. XPS and TEM provided chemical and structural details of the solid electrolyte interphase. For the cathodes, advanced imaging tools such as TOF-SIMS and HR-TEM revealed how different additive levels affected structural stability and degradation.
This multi-pronged approach allowed the team to connect performance results with underlying mechanisms. They could see not only that certain conditions worked, but why they worked, and what went wrong when the additive concentration was too high or too low.
What They Found
The results showed that the behavior of the system depends heavily on additive concentration. At 0% FEC, PTFE binders decomposed readily, leading to unstable electrode structures, severe swelling, and rapid performance loss. At 20% FEC, the electrolyte became too viscous, slowing down lithium-ion transport. Excessive FEC also produced high levels of hydrofluoric acid, which attacked the Ni-rich cathodes, causing microcracks and surface degradation.
The optimal balance was found at 10% FEC. At this concentration, a thin, stable LiF-based solid electrolyte interphase formed on the anode. This SEI suppressed PTFE decomposition, reduced volume swelling, and improved mechanical stability. At the same time, the cathode remained largely intact, avoiding the structural damage seen at higher additive levels. The result was a battery that combined high capacity with long-term stability. Full-cell testing confirmed this: the FA10 system achieved a coulombic efficiency of 99.9 percent, a capacity retention of 74.5 percent after 300 cycles, and a high areal capacity of 4.6 mAh cm−2.
Achieve consistent slurry coatings with ease using the Laboratory Roll-to-Roll Coater.
Future Outlook
The implications of this study are broad. For one, it offers a clear roadmap for making dry-processed silicon anodes viable. By demonstrating that performance can be stabilized with the right electrolyte chemistry, the study reduces one of the biggest risks of adopting silicon and dry processing in mass production. It also highlights the importance of holistic design in batteries. Additives cannot be chosen based only on their effects at one electrode. They must be optimized for the whole system. The finding that 10% FEC stabilizes both electrodes while higher concentrations harm the cathode is a perfect example of this balance.
For the industry, these results point toward a future where dry-processed silicon anodes could be adopted in EV batteries. This would bring higher energy density and greener manufacturing to large-scale battery production. If combined with roll-to-roll processing, as many companies are already exploring, the pathway to commercialization becomes even clearer.
Conclusion
This study shows that by tailoring the concentration of fluoroethylene carbonate in the electrolyte, it is possible to achieve stable, high-capacity performance in dry-processed silicon anodes paired with Ni-rich cathodes. The optimized FA10 system suppresses binder decomposition, stabilizes silicon expansion, and protects the cathode from degradation. The result is a battery that achieves high coulombic efficiency, long-term cycling stability, and high areal capacity. For the broader industry, this work provides a guideline for integrating silicon and dry processing into scalable, next-generation lithium-ion batteries.
Your coating process is only as good as the slot-die head that delivers it. Our guide helps you evaluate your needs and choose the right slot-die head for optimal quality, efficiency, and cost-effectiveness. Read the selection guide here.
Authors
Seungmin Han
Woojin Jeong
Ryeowon Kang
Minseok Kim
Mikang Jeong
Hyun-Wook Lee
Ye-Jin An
Ho-Jeong Ji
Moonsu Yoon
Patrick Joohyun Kim
Seho Sun
Taeseup Song
Dongsoo Lee
Won-Jin Kwak
Junghyun Choi
Get Professional Support for Your Battery Coating Needs
Need help with slot-die coating, coating machines, or any related applications?
Contact infinityPV’s experts today for professional guidance and support.



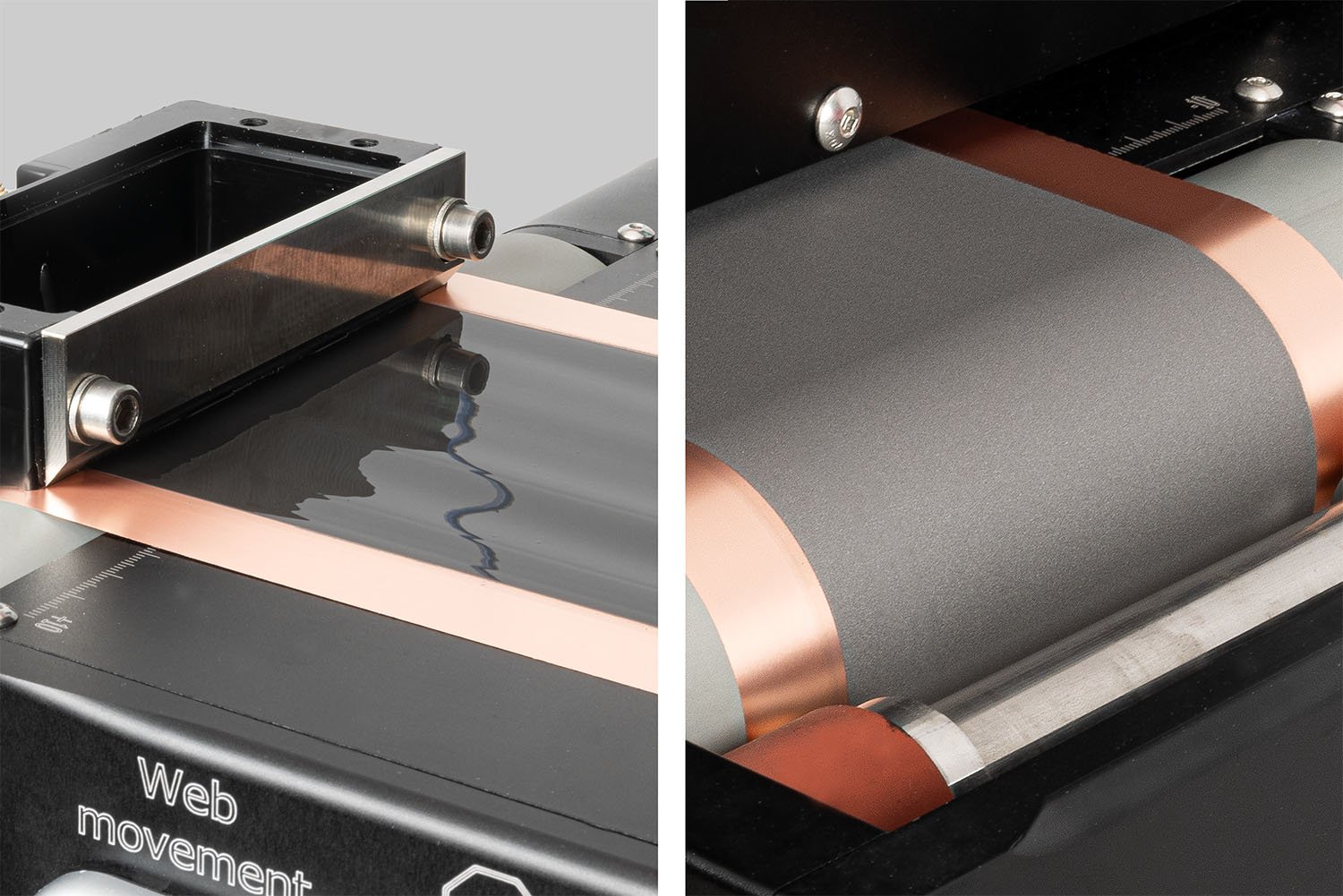





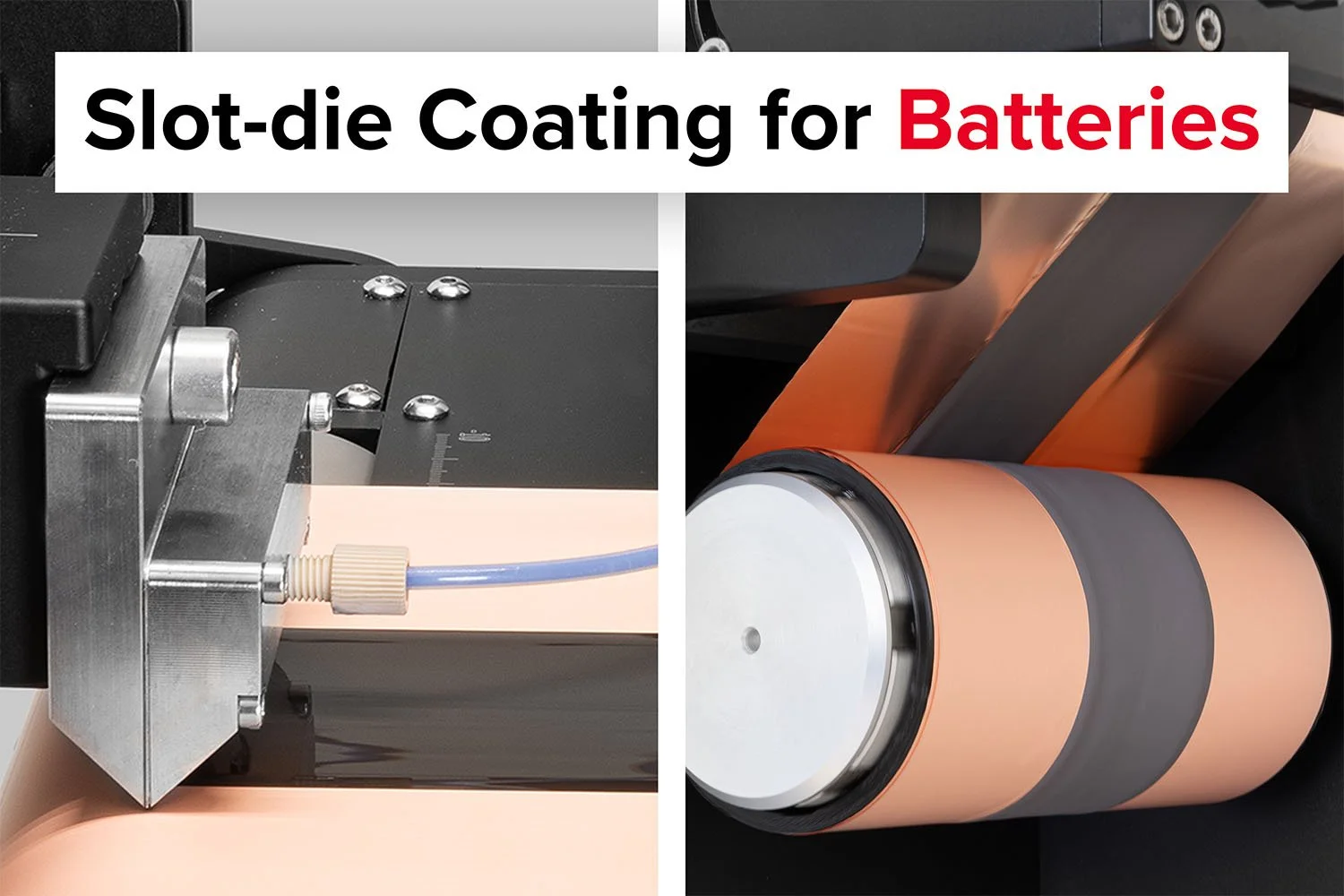















The SDC Battery Coater Pro is specifically designed for researchers dedicated to developing and optimizing battery materials. It facilitates a seamless transition from research to commercialization. View video.