Solid-State Batteries Are About to Get a Lot Safer. Here’s Why.
Solid-state batteries have long been hailed as the next big leap in energy storage. They promise higher energy density, improved safety, and longer lifespans compared to traditional lithium-ion batteries. But there’s a catch: dendrites. These tiny, tree-like metal structures grow inside batteries during charging, piercing through the electrolyte and causing short circuits or even fires.
A recent review published in the Journal of Alloys and Compounds, titled “Single-ion conducting biopolymer electrolyte films for dendrite-free solid-state lithium and sodium batteries,” reveals how scientists are turning to biopolymers, natural materials like chitosan, cellulose, and alginate, to solve this problem. These materials, derived from sources like shrimp shells, seaweed, and plant fibers, are not only sustainable but also capable of suppressing dendrites and improving battery performance.
Key Highlights
Single-ion conducting biopolymer electrolytes achieve near-perfect ion transport, effectively eliminating dendrite formation.
Biopolymers like chitosan and cellulose are biodegradable, abundant, and significantly cheaper than synthetic alternatives.
These electrolytes demonstrate impressive performance, with ionic conductivities reaching up to 1.1 × 10⁻³ S/cm and mechanical strengths of 2–4 GPa.
Scalable manufacturing methods, such as roll-to-roll processing, make these materials practical for mass production.
Biopolymer-based systems reduce CO₂ emissions by 60% compared to traditional synthetic electrolytes, offering a greener alternative.
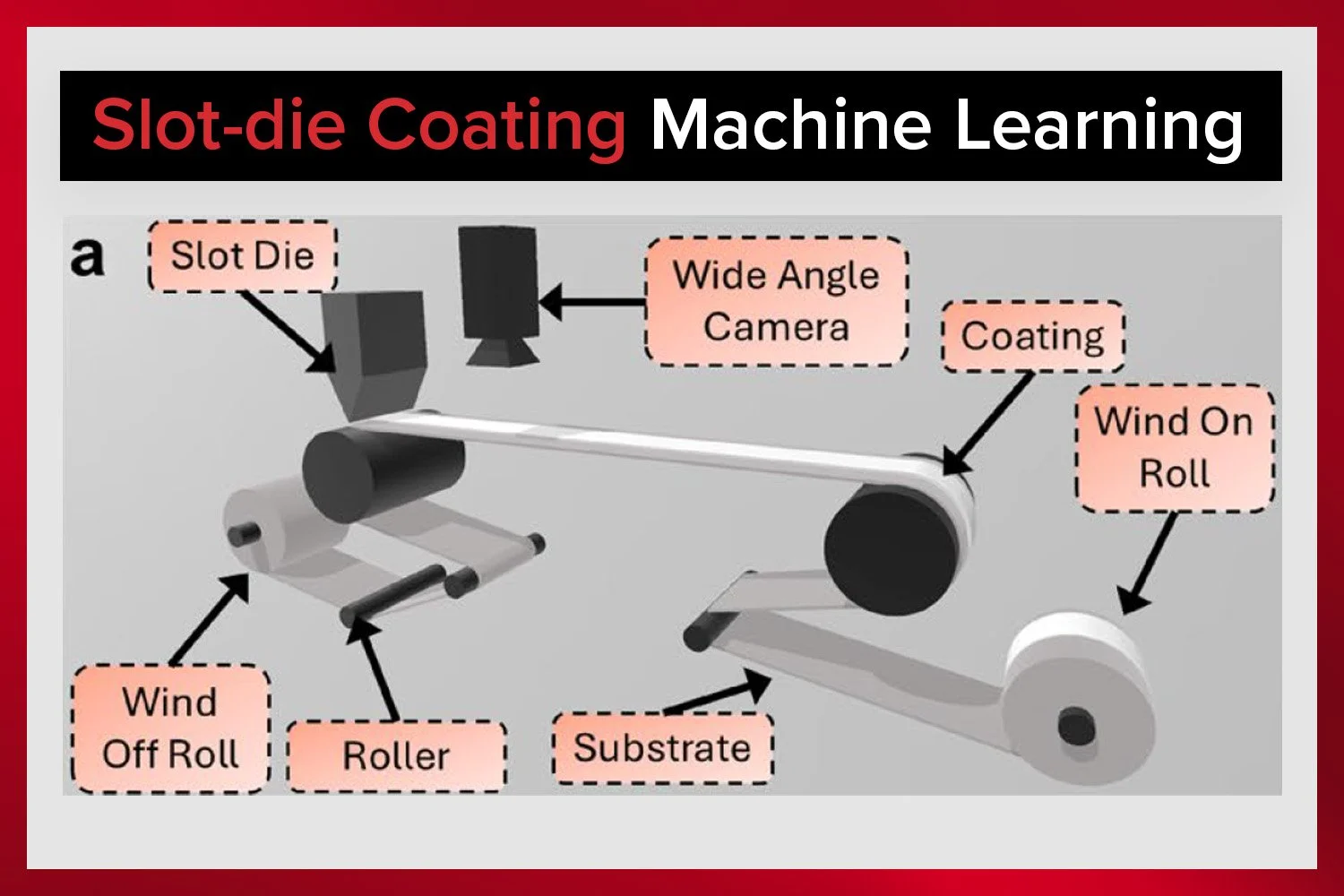
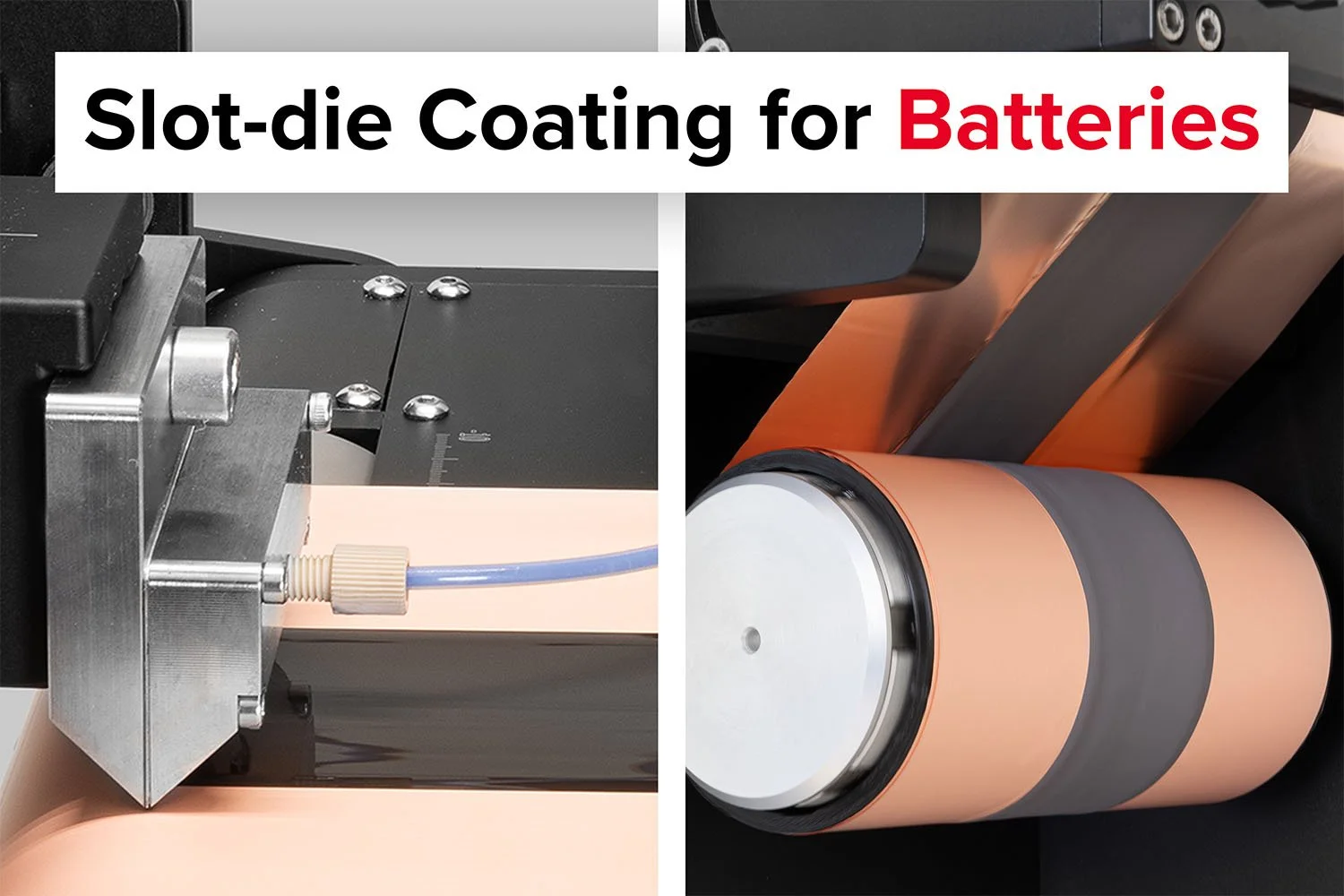
Slot-die coating is a critical step in manufacturing high-performance thin-film batteries.
The Dendrite Problem
Understanding the Challenge
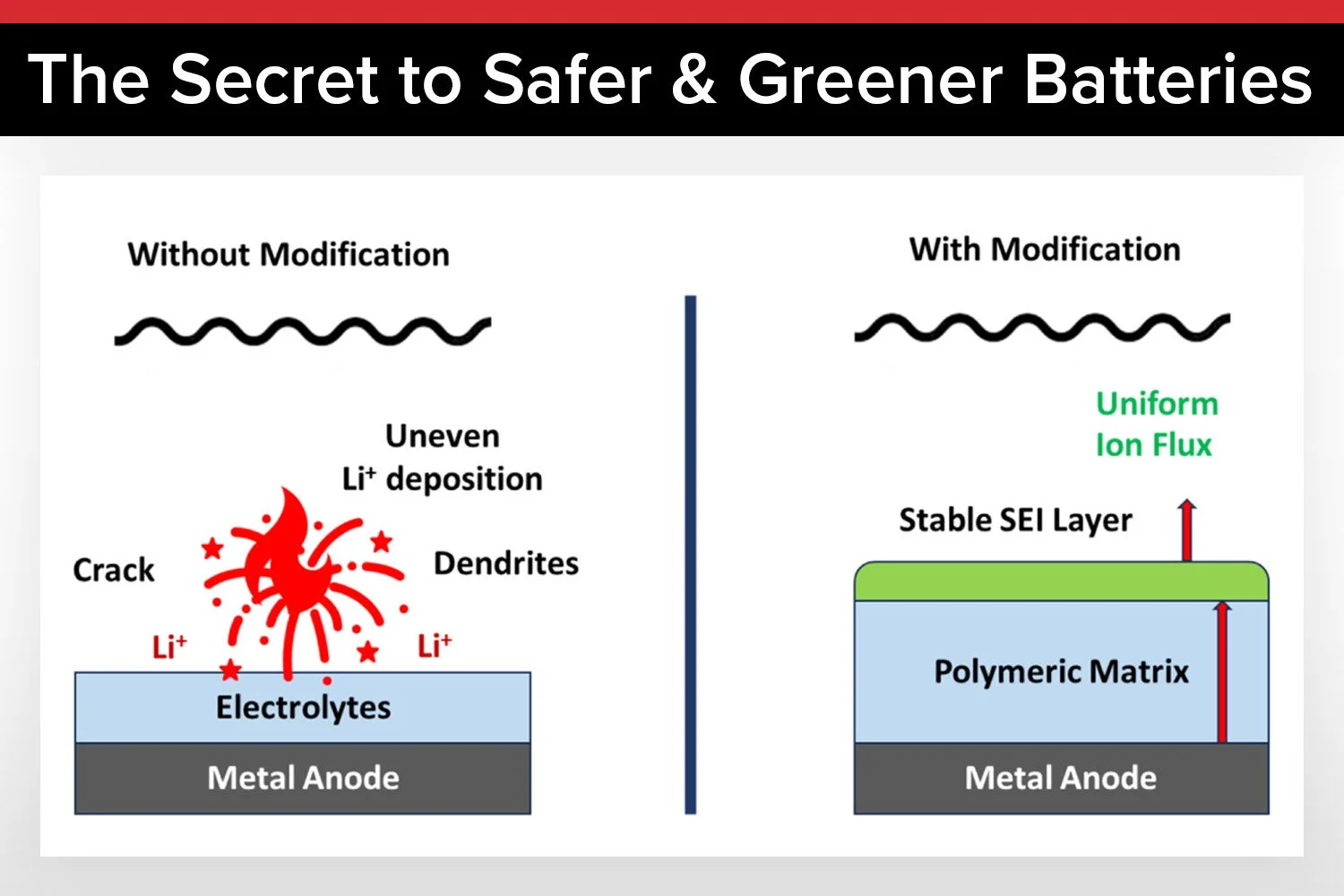
Dendrites are a persistent issue in battery technology. When a battery charges, lithium or sodium ions move from the cathode to the anode. In liquid electrolytes, these ions don’t always distribute evenly, leading to the formation of dendrites, microscopic metal spikes that grow toward the cathode. If they bridge the gap between the anode and cathode, they create a short circuit, which can cause the battery to fail or, in worst-case scenarios, catch fire.
Solid-state batteries were developed to address this issue by replacing flammable liquid electrolytes with solid materials. However, dendrites can still form due to mechanical stress, defects, or uneven ion distribution. Traditional solid electrolytes, such as ceramics or synthetic polymers, often struggle with brittleness, high production costs, or limited scalability.
How Single-Ion Conducting Electrolytes Offer a Solution
Single-ion conducting electrolytes, or SICEs, provide a clever workaround. Unlike conventional electrolytes, where both positive and negative ions move freely, SICEs immobilize the negatively charged anions while allowing only the positively charged cations—like lithium or sodium—to migrate. This eliminates the concentration gradients that drive dendrite formation.
In biopolymer-based SICEs, anionic groups such as sulfonate or carboxylate are chemically bonded to the polymer backbone. This ensures that only the desired ions move, leading to uniform ion distribution and stable battery operation, even at high current densities.
Achieve consistent slurry coatings with ease using the Laboratory Roll-to-Roll Coater.
The Power of Biopolymers in Battery Technology
Why Nature-Derived Materials Are a Game Changer
Biopolymers are polymers produced by living organisms. They include materials like chitosan, derived from crustacean shells, cellulose from plant fibers, alginate from seaweed, and starch from crops. These materials are not only abundant and biodegradable but also non-toxic, making them ideal candidates for sustainable battery technologies.
Unlike synthetic polymers, biopolymers can be chemically modified, or functionalized, to enhance their properties. For example, sulfonation adds sulfonate groups to improve ion dissociation, while phosphorylation introduces phosphate groups to boost sodium ion conduction. These modifications increase ionic conductivity, mechanical strength, and compatibility with metal anodes, making biopolymers a versatile and effective choice for battery electrolytes.
How Functionalization Enhances Performance
Functionalization is the process of chemically altering biopolymers to improve their performance as electrolytes. Techniques like sulfonation, phosphorylation, and carboxylation introduce specific groups that enhance ion transport and mechanical stability.
Sulfonation, for instance, adds sulfonate groups that improve the dissociation of lithium or sodium ions, leading to higher ionic conductivity. Phosphorylation introduces phosphate groups that are particularly effective for sodium ion conduction. Carboxylation, on the other hand, attaches carboxylate groups, offering a balanced approach to performance enhancement.
These chemical modifications transform biopolymers into highly efficient electrolytes, capable of supporting stable, high-performance battery operation.
The Laboratory Roll-to-Roll Coater makes double-sided slot-die coating simple.
The Science Behind Biopolymer SICEs
How Ion Transport Works in Biopolymer Electrolytes
In biopolymer SICEs, ion transport relies on the movement of polymer chains, a process known as polymer segmental motion. This motion allows ions to "hop" from one coordination site to another within the polymer matrix. The glass transition temperature, or Tg, is a critical factor here. Lower Tg values indicate more flexible polymer chains, which in turn lead to higher ionic conductivity.
Lithium ions, due to their small size and high charge density, form strong coordination bonds with anionic groups in the polymer. Sodium ions, while larger and less strongly bound, benefit from faster ion exchange kinetics. Both types of ions require optimized functionalization to ensure stability and performance.
Mechanical and Electrochemical Stability
For dendrite suppression, electrolytes need to meet several key criteria. They must have a high shear modulus—greater than 1 GPa for sodium and over 6 GPa for lithium—to prevent dendrite penetration. They also need a wide electrochemical window, exceeding 4.5V, to ensure stability at high voltages, and thermal stability up to 150°C to prevent degradation during operation.
Biopolymer SICEs achieve these properties through nanocomposite reinforcement. By adding fillers like LLZTO, a ceramic conductor, or MXene, a two-dimensional material, researchers enhance both conductivity and mechanical strength. These reinforcements create a robust electrolyte that can withstand the demands of real-world battery applications.
Roll-to-roll processing is perfect for scaling up fuel cell production.
Why This Review Matters for the Battery Industry
Bridging the Gap Between Research and Commercialization
This review is more than just a scientific exploration—it’s a blueprint for bringing biopolymer electrolytes to market. It highlights scalable fabrication methods, such as roll-to-roll slot-die coating, which can produce uniform electrolyte films on a large scale. It also underscores the cost-effectiveness of biopolymers, which are significantly cheaper than synthetic alternatives, with materials like starch and cellulose costing as little as $1–3 per kilogram.
Beyond cost and scalability, biopolymer SICEs offer substantial environmental benefits. They reduce CO₂ emissions by 60% compared to traditional electrolytes, aligning with global efforts to develop greener, more sustainable technologies.
Applications Beyond Traditional Batteries
The potential of biopolymer SICEs extends far beyond conventional battery applications. Their flexibility and biocompatibility make them ideal for flexible and wearable electronics, where traditional rigid batteries fall short. They also hold promise for grid storage and electric vehicles, where safety and longevity are paramount.
Looking ahead, hybrid systems that combine lithium and sodium ion conduction could offer the best of both worlds—balancing performance, cost, and sustainability. These innovations could redefine energy storage, making it safer, more efficient, and more accessible.
Your coating process is only as good as the slot-die head that delivers it. Our guide helps you evaluate your needs and choose the right slot-die head for optimal quality, efficiency, and cost-effectiveness. Read the selection guide here.
The Future of Biopolymer Electrolytes
Emerging Trends and Innovations
The field of biopolymer electrolytes is rapidly evolving, with several exciting trends on the horizon. Artificial intelligence and machine learning are accelerating the discovery of new biopolymer-electrolyte combinations by predicting ion transport properties and optimizing material designs. Hybrid systems that leverage both lithium and sodium ions are also gaining traction, offering a balanced approach to performance and cost.
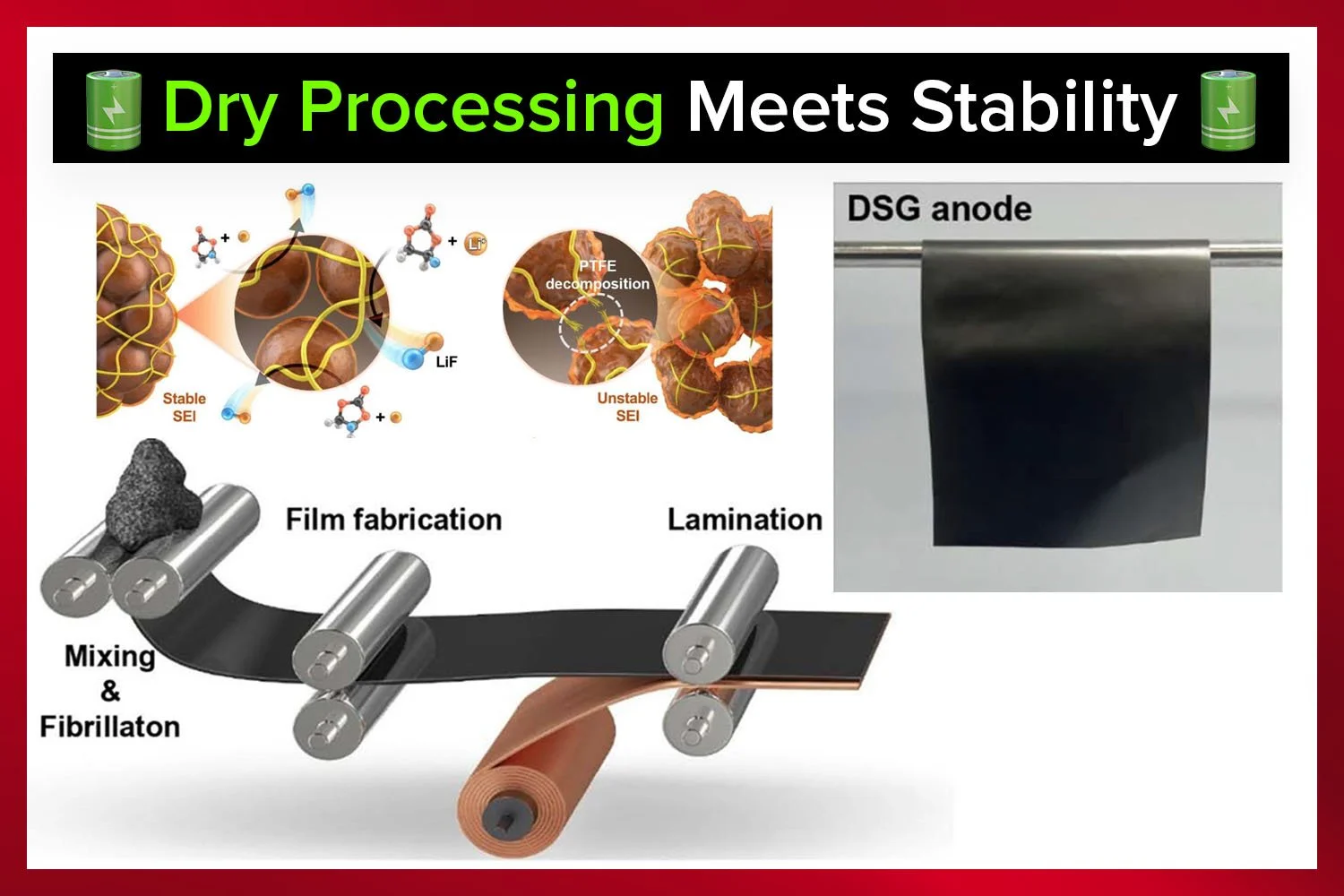
Self-healing electrolytes represent another promising innovation. By using dynamic crosslinking, these materials can repair mechanical damage, extending battery life and improving reliability. Green manufacturing practices, such as water-based processing and biodegradable materials, are further enhancing the sustainability of these technologies.
Challenges to Overcome
Despite the progress, challenges remain. Moisture sensitivity is a significant issue, as biopolymers can absorb water, which degrades performance. Solutions like hydrophobic coatings and hermetic packaging are being explored to mitigate this problem.
Interfacial stability—ensuring long-term compatibility between electrolytes and electrodes—is another critical area of focus. Scalability, particularly optimizing roll-to-roll processes for high-volume production, will also be key to bringing these technologies to market.
A Sustainable Path Forward
The review “Single-ion conducting biopolymer electrolyte films for dendrite-free solid-state lithium and sodium batteries” presents a compelling vision for the future of energy storage. By harnessing the power of natural materials and advanced functionalization techniques, researchers have developed electrolytes that suppress dendrites, enhance performance, and reduce environmental impact.
For industries looking to adopt next-generation battery technologies, biopolymer SICEs offer a scalable, cost-effective, and eco-friendly solution. As research continues to advance, we can expect to see these materials in everything from electric vehicles to wearable devices, paving the way for a cleaner, safer, and more sustainable energy future.
Authors
Aravindh Murali
C. Naveen
Get Professional Support for Your Battery Coating Needs
Need help with slot-die coating, coating machines, or any related applications?
Contact infinityPV’s experts today for professional guidance and support.

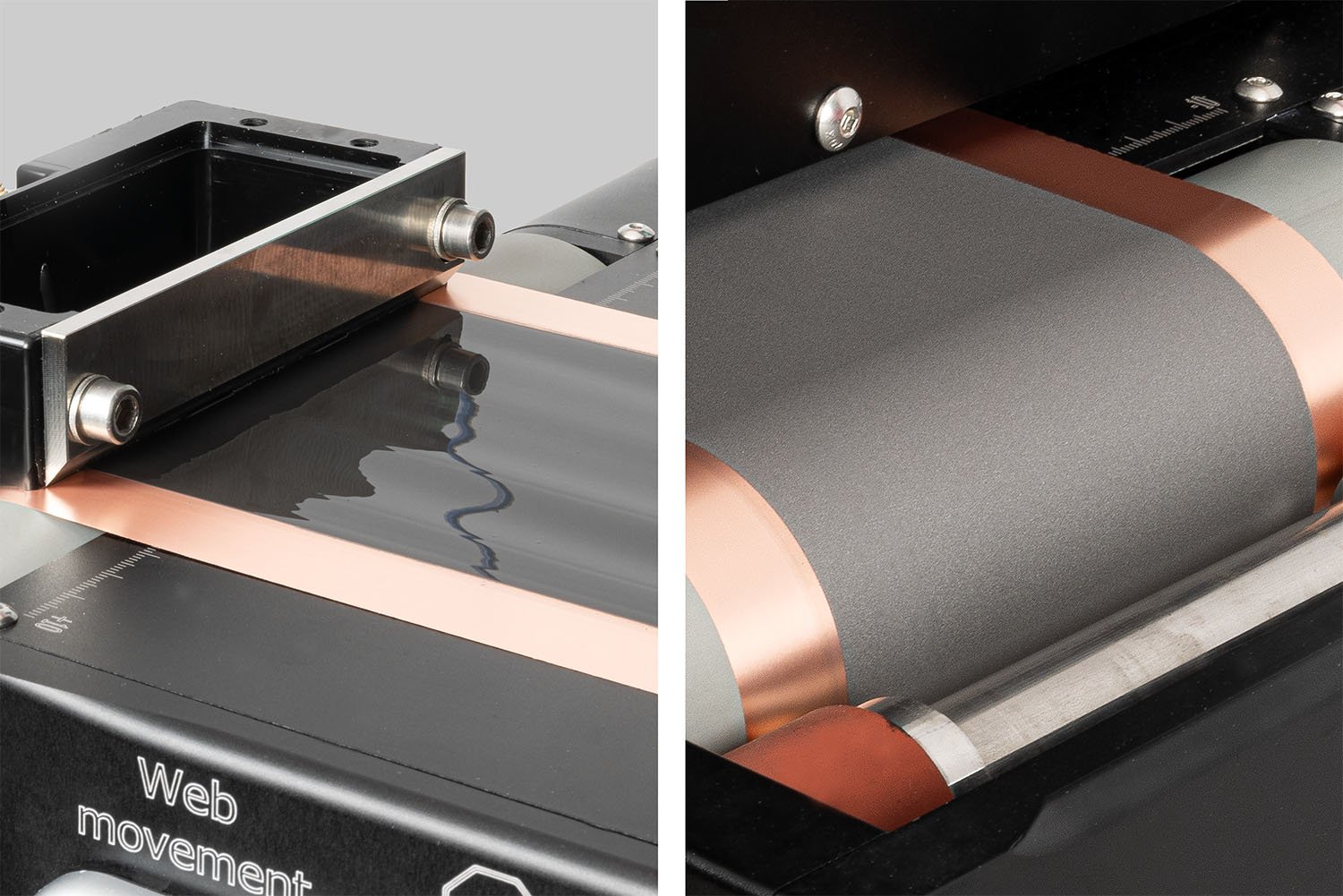


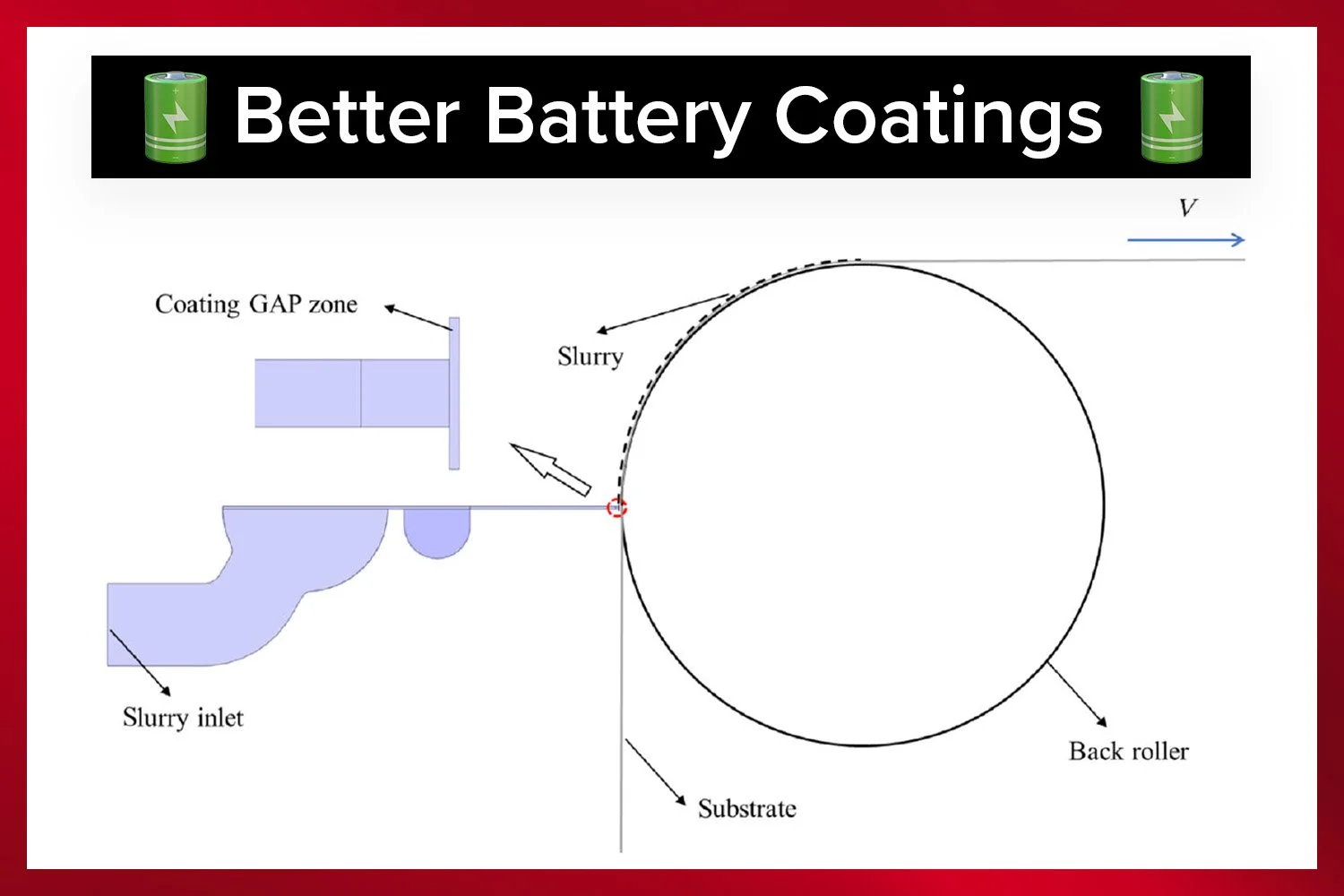



















The SDC Battery Coater Pro is specifically designed for researchers dedicated to developing and optimizing battery materials. It facilitates a seamless transition from research to commercialization. View video.